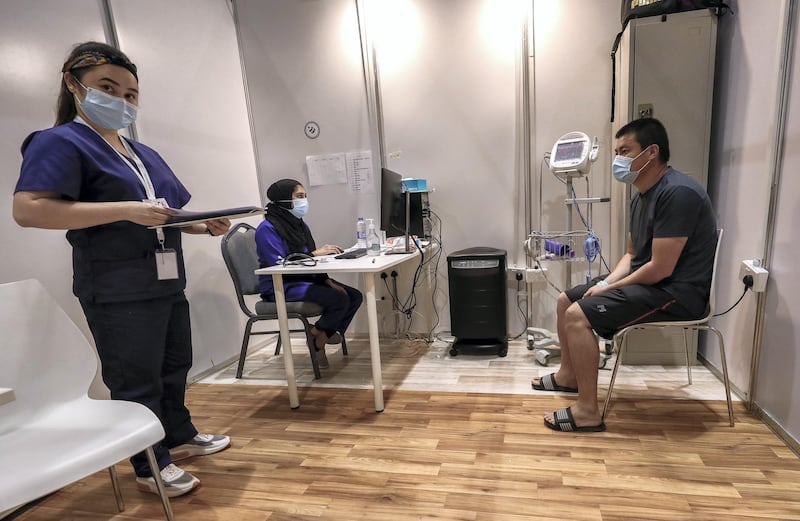
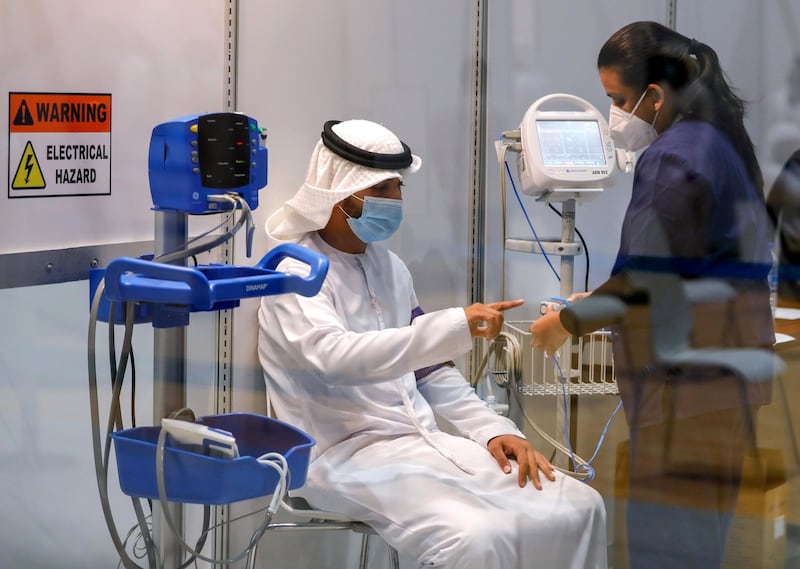
A Covid-19 vaccine trial being run in Abu Dhabi will expand to Bahrain in the search for more volunteers.
A centre will be set up in the kingdom as part of the UAE-China project to test Chinese drug maker Sinopharm's potential vaccines on large numbers of people.
Abu Dhabi healthcare company G42, which announced the move, said it sought up to 6,000 volunteers in Bahrain, following the opening of volunteer centres in Abu Dhabi and Sharjah.
Medics are looking for volunteers aged between 18 and 60 in Bahrain and the trial will last for six to 12 months.
In total, 15,000 volunteers are sought for the Sinopharm trial, which began in mid-July.
“It was always part of our original plan to open several centres to ensure the broadest impact and opportunity for individuals to participate and join the 4Humanity campaign," said Ashish Koshy, chief executive of G42 Healthcare, an artificial intelligence and data company, which is working on the trial with Abu Dhabi's health authorities.
"There has been a hugely enthusiastic response from the Ministry of Health and other public health bodies in Bahrain to work with us on the trials and to encourage their communities to volunteer in the trials."
Phase III trials involve the testing of the potential vaccine on large numbers of people to determine if it is effective.
Volunteers receive two vaccine shots in one month and then are monitored for up to 12 months afterwards, keeping a diary to record any observations or side effects.
To date, more than 5,500 people in the UAE have received the shots, including Sheikh Abdullah bin Mohammed Al Hamed, chairman of the Department of Health Abu Dhabi, and Dr Jamal Al Kaabi, acting undersecretary of the department, who is overseeing the trial.
Medics look for people from a wide variety of ages, backgrounds and ethnicities as part of the process - one of the reasons the UAE was chosen.
“The expansion will also help to boost the overall numbers of people participating in the test to enable similar numbers to other international trials underway in nations with much larger populations," said Mr Koshy.
"We are also anticipating an expansion of the our centre network in the UAE to be announced shortly.”
Sinopharm's Phase I and Phase II trials were regarded as successful and results in 100 per cent of volunteers generating antibodies for Sars-CoV-2, the coronavirus that causes Covid-19, after two shots in 28 days.
On Monday, Saudi Arabia said it would begin a trial of a separate vaccine developed by China's CanSino Biologics.
On Tuesday, Russia's President Vladimir Putin said the country had given regulatory approval to the world's first vaccine after two months of human testing, though the final stages of clinical trials to test safety and efficacy continue.
Volunteers for the Abu Dhabi trial can register at 4humanity.ae or by calling 02 819 1111.