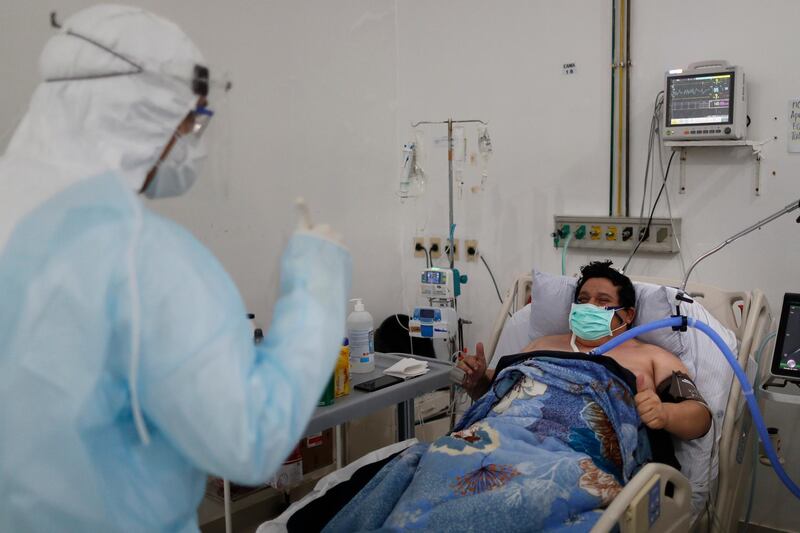
US President Donald Trump announced emergency authorisation to treat Covid-19 patients with convalescent plasma – a move he called “a breakthrough”.
One of Mr Trump's top health officials called the measure “promising” and other health experts said it needed more study before being celebrated.
The announcement on Sunday came after White House officials complained there were politically motivated delays by the Food and Drug Administration in approving a vaccine and therapeutics for the disease that has reversed Mr Trump’s re-election chances.
On the eve of the Republican National Convention, Mr Trump put himself at the centre of the FDA’s announcement of the authorisation at a news conference on Sunday evening. The authorisation makes it easier for some patients to obtain the treatment but is not the same as full FDA approval.
The blood plasma, taken from patients who have recovered from the coronavirus and rich in antibodies, may provide benefits to those battling the disease. But the evidence so far has not been conclusive about whether it works, when to administer it and what dose is needed.
In a letter describing the emergency authorisation, the chief scientist for the FDA, Denise Hinton, said: “Covid-19 convalescent plasma should not be considered a new standard of care for the treatment of patients with Covid-19. Additional data will be forthcoming from other analyses and ongoing, well-controlled clinical trials in the coming months.”
But Mr Trump had made clear to aides that he was eager to show good news in the battle against the virus, and the timing allowed him to head into his convention with momentum. He and aides billed it as a “major” development and used the White House briefing room to make the announcement.
Mr Trump also displayed some rare discipline in the evening news conference, sticking to his talking points, deferring to the head of the FDA, Stephen Hahn, and only taking three questions from reporters.
The White House had grown agitated with the pace of the plasma approval. The accusations of an FDA slowdown, which were presented without evidence, were just the latest assault from Mr Trump’s team on what he refers to as the “deep state” bureaucracy. White House chief of staff Mark Meadows did not deal in specifics, but said that “we’ve looked at a number of people that are not being as diligent as they should be in terms of getting to the bottom of it”.
On Saturday, Mr Trump criticised the process to treat the virus, which has killed more than 180,000 Americans and endangered his re-election chances.
The White House has sunk vast resources into an expedited process to develop a vaccine and his aides have been banking on it being an “October surprise” that could help him to make up ground in the polls.
“The deep state, or whoever, over at the FDA is making it very difficult for drug companies to get people in order to test the vaccines and therapeutics,” Mr Trump said Twitter.
“Obviously, they are hoping to delay the answer until after November 3. Must focus on speed and saving lives.”
This month, Mayo Clinic researchers reported a strong hint that blood plasma from Covid-19 survivors helps other infected patients to recover, but it was not considered to be proof.
More than 64,000 patients in the US have been given convalescent plasma, a century-old approach to fend off influenza and measles before vaccines.
History suggests that it works against some, but not all, types of infection.
The Mayo Clinic reported preliminary data from 35,000 coronavirus patients treated with plasma.
It said there were fewer deaths among people given the treatment within three days of diagnosis, and among those given plasma with the highest levels of virus-fighting antibodies.
But it was not a formal study. The patients were treated in different ways in hospitals around the country as part of an FDA programme to speed up access to the experimental therapy.
The study cannot prove the plasma, and not other care they received, was the reason for improvement.
There has been little data on how effective it is or whether it must be administered early in an illness to make a significant difference, said Dr William Schaffner, an infectious diseases expert at Vanderbilt University in the US.
Rigorous studies under way around the country are designed to obtain that proof, by comparing similar patients randomly assigned to have plasma or a dummy infusion in addition to regular care.
But those studies have been difficult to finish as the virus surges and falls in different cities.
Meanwhile, former FDA commissioner Scott Gottlieb dismissed the suggestion of a slowdown.
“I firmly reject the idea they would slow-walk anything or accelerate anything based on any political consideration or any consideration other than what is best for the public health and a real sense of mission to patients,” Mr Gottlieb told CBS.
Hundreds of drugs are being developed as possible treatments against the coronavirus, in a range of approaches.
Mr Trump “has made all kinds of therapeutic suggestions” that have not proven to be supported by science and are even dangerous, Mr Schaffner said.
That included statements about the possible value of treating Covid-19 patients with ultraviolet light and bleach.
But Mr Trump is best known for his early and ardent embrace of the antimalarial drugs hydroxychloroquine and chloroquine.
The FDA in late March granted emergency authorisation for distribution of the drugs for treating Covid-19.
But in June, the agency revoked the authorisation after growing evidence that they did not work and could cause serious side effects.
The FDA also warned doctors against prescribing the drugs in combination with remdesivir, which was shown to help patients with Covid-19.
The FDA said the anti-malarial drugs could reduce the effectiveness of remdesivir, which the FDA cleared for emergency use in May.
A top FDA official who is overseeing Covid vaccine trials vowed to resign if the Trump administration approved a vaccine before it was shown to be safe and effective.
Peter Marks, director of the Centre for Biologics Evaluation and Research, made his promise this month in a conference call with pharmaceutical executives, government officials and others, Reuters reported on Friday.