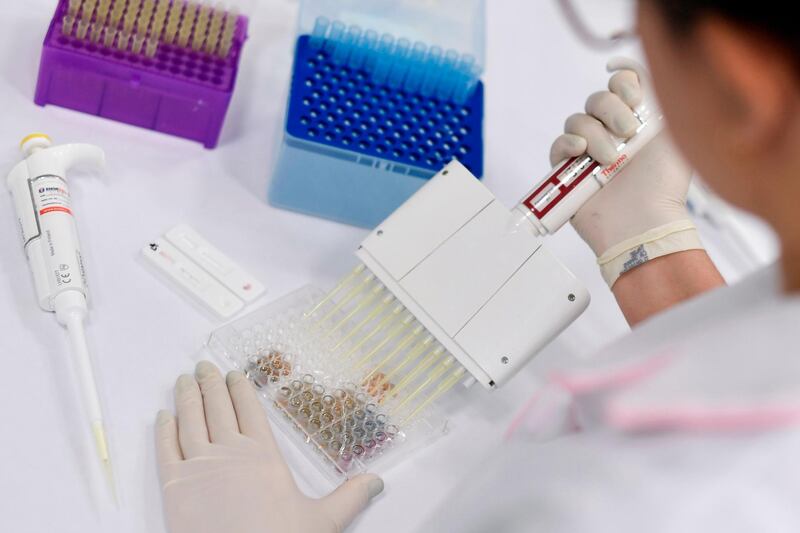
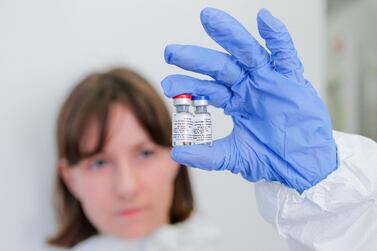
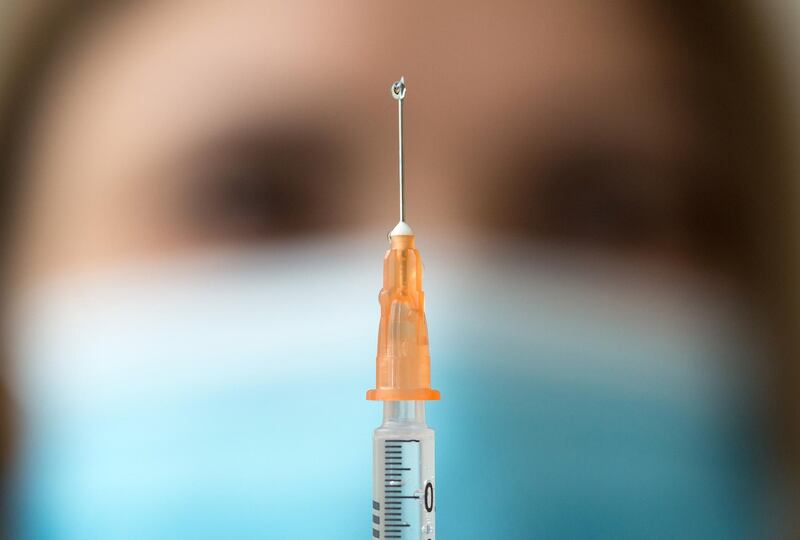
Britain has signed two deals to purchase doses of experimental vaccines for Covid-19, bringing its basket of pre-supply deals with drug companies to six.
Johnson & Johnson said its Janssen Pharmaceutica would supply the UK with its candidate, known as Ad26.COV2.S, with an initial sale of 30 million doses on a non-profit basis for emergency pandemic use.
The advance purchase agreement will also provide an option for an additional purchase of up to 22 million doses, it said.
In a separate statement, Novavax said the UK would buy 60 million doses of its coronavirus vaccine candidate, NVX-CoV2373, for a phase 3 clinical trial.
With six deals each so far, Britain and the United States are well placed in the global race to strike deals with drugmakers for vaccines as the pandemic continues to rage. Russia has said it hopes to be the first to roll out a vaccine programme with its own candidate within weeks.
The latest agreements bring the total number of potential vaccine doses secured by the UK to more than 300 million. The country of 66 million people has also signed deals with partners GlaxoSmithKline and Sanofi, Pfizer and BioNTech SE as well as Valneva SE.
The University of Oxford and AstraZeneca intend to make as many as 30 million doses available by September as part of a pact to deliver 100 million doses.
Britain also agreed to help both Novavax and Janssen conduct third-phase clinical trials. Novavax should start in the third quarter, the company said. Novavax has an alliance with Fujifilm Diosynth Biotechnologies that will produce as many as 180 million doses of the vaccine annually in north-east England.
The European Commission said late on Thursday that it was seeking to secure 200 million vaccine doses from J&J. The European Union has also said it was closing in on a deal for as many as 300 million doses of the shot that Sanofi and Glaxo were developing.
Results presented this month from a two-injection regimen of Novavax’s vaccine, when administered with the company’s immune-boosting technology, generated antibody responses that were four times higher than those seen in people who had recovered from the disease.
The company has secured a $1.6 billion (Dh5.88bn) contract from the US government under its Operation Warp Speed programme.