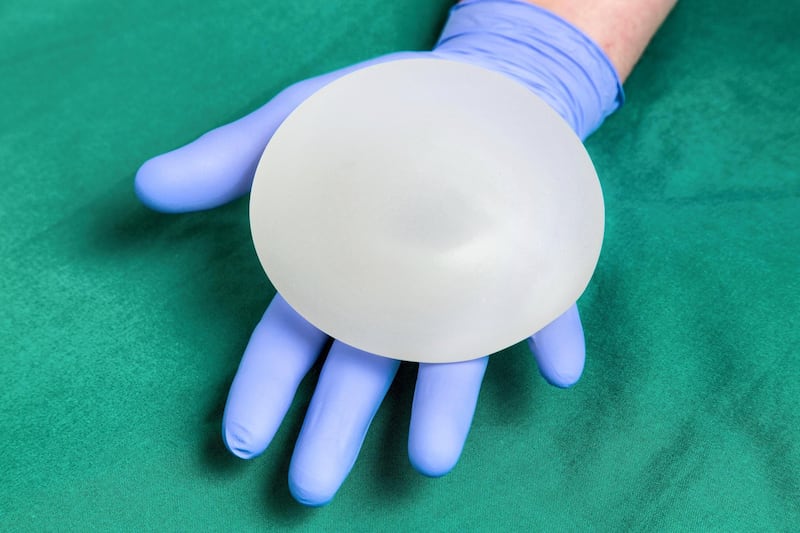
UAE surgeons are withdrawing a model of breast implant from use after new evidence showed a heightened risk of cancer.
The Irish pharmaceutical company Allergan ordered a worldwide recall of their Biocell implants this week after US authorities linked them to the vast majority of cases of a rare form of lymphoma.
The implants have a textured surface designed to prevent slippage and minimise scar tissue. The recall does not affect the Irish manufacturer's smooth implants nor a separate textured model called Microcell.
Dr Luiz Toledo, a cosmetic surgeon in Dubai and the International Society of Plastic Surgery's national secretary for UAE members, said that despite the alert, women with the implants will not need to have them removed.
"If a fault is detected in the implant then can be repaired," she said. "It is solvable."
He said BioCell implants are used in many markets, including in the UAE.
"I have a few patients wanting to know what implants they have. I imagine that people will want to know," he said.
The US Food and Drug Administration said the latest figures show more than 80 per cent of the 570 confirmed cases of the lymphoma worldwide have been linked to Allergan implants. Regulators estimate that the risk of the disease is six times higher with Allergan than other textured implants sold in the US.
There is no firm agreement on the exact frequency of the disease, known as breast implant-associated anaplastic large cell lymphoma.
Published estimates range from 1 in 3,000 patients to 1 in 30,000 patients.
Dr Toledo said he would caution against any panic and said the recall is not comparable to the early 1990s silicone implant scandal.
"These cases are very rare – some estimates say 1 in every 100,000 – and it is solvable," he said.
Allergan said it would no longer sell or distribute Biocell implants and tissue expanders, which are used to prepare patients for breast reconstruction. The company said surgeons should return unused implants.