Members of the public in Abu Dhabi and Al Ain can now register to take part in a clinical trial aimed at finding a vaccine for Covid-19.
Officials are hoping 15,000 people living in the two cities can be persuaded to volunteer for three to six months.
Almost anyone between the ages of 18 and 60 is able to sign up provided they pass a thorough medical exam.
Volunteers can register on the government website 4humanity.ae or by ringing 02 819 1111.
“Your participation in these vital Phase-III trials can play a massive role in helping to overcome the global Covid-19 pandemic,” a statement on the website reads.
“In the weeks ahead, as you help us in this important initiative, we wanted to reassure you that your safety will always come first.”
The Phase-III clinical trial is being conducted by Abu Dhabi Health Services Company at five sites in the capital and Al Ain.
It is the first to be recognised by the World Health Organisation and represents the final phase of study prior to obtaining regulatory permission to manufacture.
Phase III trials typically involve evaluating the effectiveness of a drug on a large population sample.
Phase IV trials involve continuing to monitor the safety of the drug after it has been prescribed to patients.
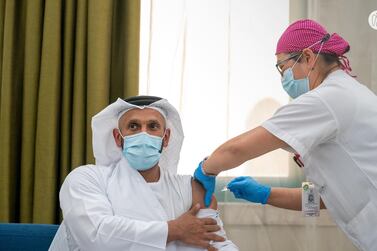
Earlier this week, Sheikh Abdullah bin Mohammed Al Hamed, chairman of the Department of Health Abu Dhabi, was the first volunteer to take part in the latest stage of the research programme.
He was followed by Dr Jamal Al Kaabi, the acting undersecretary of the same department.
Scientist hope the trial will help determine specific antibodies that can fight the virus and provide immunity.
Anyone suffering from chronic illnesses, or who is pregnant, cannot take part.