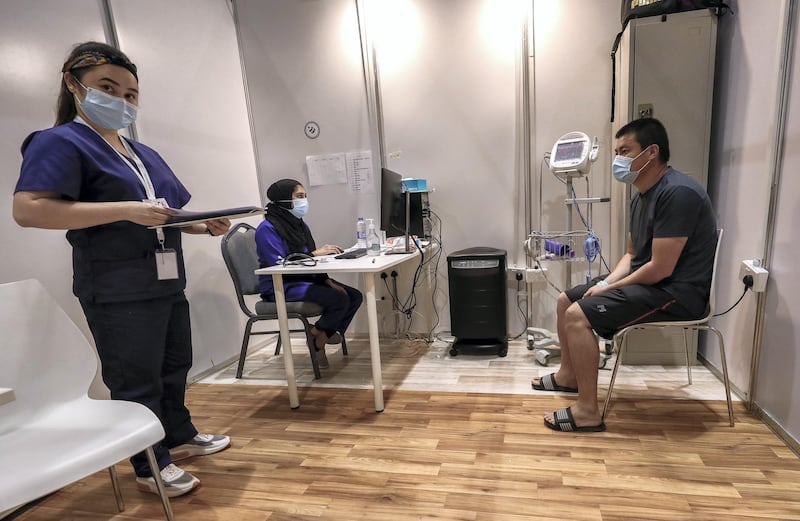
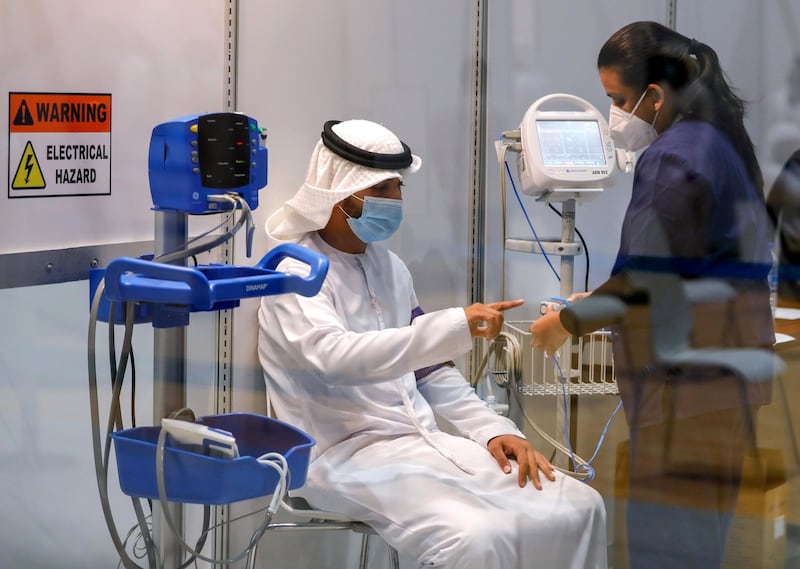
More than 15,000 volunteers have registered for a Covid-19 vaccine clinical trial in the UAE, authorities said on Thursday.
Volunteers from 107 nationalities signed up to test the inactive vaccine, which contains a killed version of Covid-19, meeting the required number of subjects for the trial.
Health officials said the 15,000 eligible volunteers had received their first of two doses and would be inoculated with the second within the next few weeks.
Dr Abdul Rahman Al Owais, Minister of Health and Prevention, said this was a proud achievement for the country.
"This is a very proud day for everyone associated with these trials and we are looking forward to continuing to play an integral role in the work to come in the months ahead," he said.
The UAE's Phase III COVID-19 Inactivated Vaccine Trial achieved its target of 15,000 volunteers within less than a month, marking a major milestone towards developing the first tested, safe vaccine. pic.twitter.com/4SLCMeiJBv
— مكتب أبوظبي الإعلامي (@admediaoffice) August 13, 2020
More than 4,500 of the 15,000 vaccinated volunteers were Emirati and around 140 doctors, 300 nurses, and many more administrative and technical support staff helped facilitate the trial.
Thousands of volunteers signed up but not all were eligible because they did not meet criteria for the testing. Eligible volunteers must have no pre-existing health conditions, including asthma, cardiovascular diseases, kidney disease or malignant tumours. They should not have experienced a fever, dry cough, fatigue, nasal obstruction or a runny nose for at least 14 days before they took the first shot.
Inside Abu Dhabi's clinic for Covid-19 vaccine trial

The first to sign up and receive both vaccinations was Sheikh Abdullah bin Mohammed Al Hamed, chairman of the Department of Health Abu Dhabi, which is helping to co-ordinate the trials.
"I was enormously proud to have been the first volunteer and since then it has been truly heartwarming to see how the nation has pulled together, helping us achieve our target of participants in just four weeks since the trials began. We are a nation of extraordinary people, one that individuals from all over the world call home, and together we are committed to finding a global solution for Covid-19," he said.
Phase 3 of the trials are being conducted in Abu Dhabi National Exhibition Centre in Abu Dhabi, Al Qarain Health Centre in Sharjah and were expanded to include a centre in Bahrain this week.
The vaccine was developed by pharmaceutical company Sinopharm, based in China, where phases 1 and 2 of the trials were successfully conducted.
The UAE is involved through an agreement with technology company Group 42 and was chosen for the third phase for its population diversity.
"The speed of these trials to date and the incredible diversity of volunteers who have been vaccinated has fully reinforced our decision that the UAE was a perfect location to test our inactivated vaccine and reach the widest demographic and ethnic range of volunteers," said Jingjin Zhu, president of Sinopharm Group Biological Products.
Moving forward, volunteers will receive their second dose and continue to undergo regular monitoring and health checks. The entire process from vaccination to completion of health checks takes 42 days.
The World Health Organisation-recognised trial began on July 16.
Hundreds of people are now volunteering for the phase 3 trials in Bahrain, which are managed by G42 Healthcare with the Bahrain Ministry of Health. Results will be part of the overall trials that began in the UAE and use the same inactivated vaccine from Sinopharm and identical clinical protocols.
To volunteer for the Abu Dhabi trial, register your details at 4humanity.ae or call 02 819 1111.