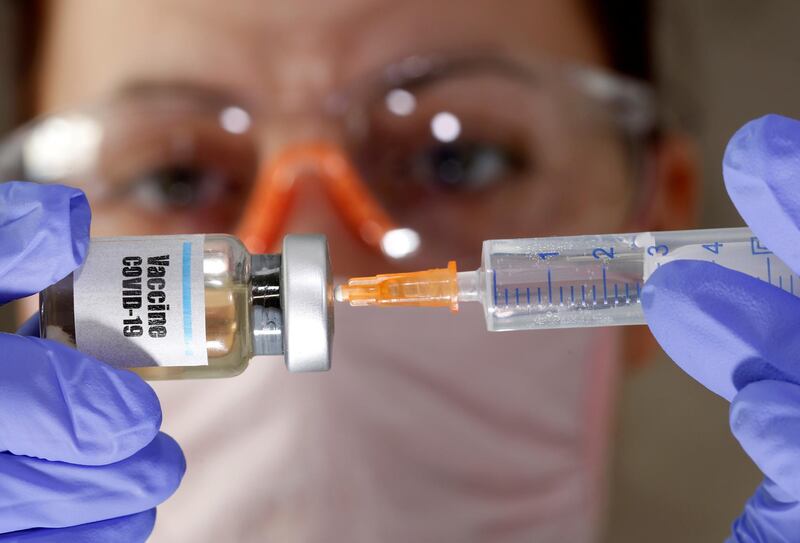
As the race to develop a vaccine and a therapy for Covid-19 hots up, the Billion Molecules Against COVID-19 Global GrandChallenge – organised by the Joint European Disruptive Initiative (JEDI) – kicked off on 4 May. At the same time, the EU – in co-operation with France, Germany, the UK, Norway and Saudi Arabia – has launched a massive fund-raising marathon. The purpose of it is twofold: to accelerate the development and deployment of tests, treatments and vaccines, and to ensure that as many players as possible undertake to make them accessible to all countries. And the EU is thinking big money, as always, it raised no less than €7.4 billion in initial funding.
How will we all access vaccines?
Although the ambition of this EU fundraising is laudable, the problem of the distribution of the vaccine to the whole world needs to be better analysed. Pharma players capable of producing the right scale of doses are rare.
In recent weeks, partnerships have multiplied between large laboratories to increase their production capacities: American biotech company Moderna and the Swiss drugmaker Lonza Group aim for a billion doses per year.
The British pharma company AstraZeneca and the University of Oxford hope to be able to supply 100 million doses before the end of the year. The American Inovio Pharmaceuticals and the German Richter-Helm Biologics have joined forces for the same purpose, as well as arch-rivals Sanofi (French) and GlaxoSmithKline (English).
But even more than the question of when and by whom a vaccine will be discovered – and the German minister of health recently stated this would not happen soon – the major challenge for humanity is the quantity of doses that can be produced and the accessibility of these doses.
Developing a vaccine also creates a possibility of returning to normal economic activity without the fear of a ’second wave’.
However, if tests conducted by American laboratories are successful, it is likely that the vaccines will go first to the American population.
What is already problematic for Europe is likely to prove disastrous for countries that have neither infrastructure, financial means nor the manpower of Western countries. The slow and expensive vaccine manufacturing process could strategically promote inequality.
A need for collective action
Tackling the challenge to ensure massive production and equitable distribution requires collective action. The World Bank and the Coalition for Epidemic Preparedness Innovations are working on the matter of financing. But the question of transforming production processes to make them more efficient is also crucial.
Intensifying the process of developing a vaccine is a possible approach, with the aim of minimising the equipment and space used. This would reduce both cost and risk and create the potential to produce the drug anywhere in the world.
Several players are already working on this idea, such as the Belgian-based Univercells and the Dutch company Batavia Pharma, but also the pharmaceutical companies Janssen and Merck, as well as the technology and service provider Cytiva. But there are numerous obstacles on the way and we need to face these scientific and industrial frontiers.
Funding is not the only solution
If the EU wishes to rise to the challenge it has launched, funding the design and production of vaccines and treatments will not be enough.
Let us use this historic and planetary crisis to imagine solutions that are both scientifically robust and radically new to develop and produce them: distributed production and 3D printing, for instance, or bringing leading scientists together and new capabilities brought by high performance computing, machine learning and molecular biology to screen billions of molecules to fight coronavirus, as we have done at the Joint European Disruptive Initiative, a unique and promising approach.
Creativity is essential
We need to fast-track drug discovery by having even better qualified compounds enter clinical trials, compounds that could be correlated by teams from across the globe. We need to tap into collective intelligence, be creative, to experiment, push the limits of science and technology and be creative.
The sovereignty of nations is as much at stake as equal access to health throughout the world. Indeed, if the coronavirus currently is monopolising our attention, it would be absurd not to prepare today for another epidemic. The number of pathogens with pandemic potential are many and some have much higher mortality rates than the coronavirus.
To get out of the current tragedy and not be at the same point when the next disease strikes, we must take seriously the call to the Europeans by the poet and writer Paul Valery: "Well, what are you going to do? What are you going to do today?"
Andre Loesekrug-Pietri is executive director of the Joint European Disruptive Initiative