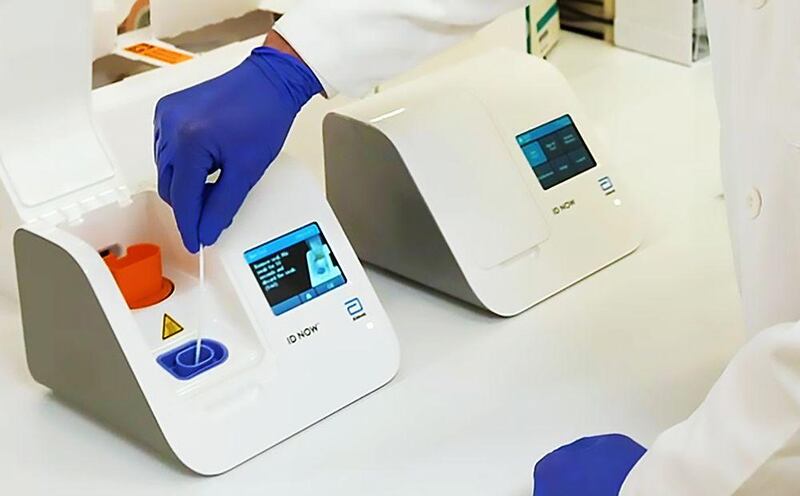
US-based Abbott Laboratories rolled out a light and portable Covid-19 testing device that can detect if a person is infected with the virus within 5 minutes and can be used in a wide range of healthcare settings.
The New York-listed health technology provider plans to provide the Covid-19 tests next week and expects to ramp up manufacturing to deliver 50,000 tests per day, it said in a statement on Friday. The test uses molecular technology and can be deployed in settings including physicians' offices, urgent care clinics and hospital emergency departments.
"The Covid-19 pandemic will be fought on multiple fronts, and a portable molecular test that offers results in minutes adds to the broad range of diagnostic solutions needed to combat this virus," Robert B. Ford, president and chief operating officer of Abbott, said.
Globally, the number of Covid-19 confirmed cases rose to about 616,000 on Saturday and the death toll reached nearly 29,000 while about 136,000 people have recovered, according to the John Hopkins University coronavirus tracker.
The US has about 105,000 coronavirus cases, the highest in the world, overtaking Italy and China. US hospitals are overwhelmed with demand for testing thousands of people for the deadly virus. The US and other countries are struggling with a shortage of testing devices and personal protection equipment for their healthcare workers on the frontlines.
The test can deliver positive results in as little as five minutes and negative results in 13 minutes, according to the company.
Abbott said it won approval from the US Food and Drug Administration (FDA) under its Emergency Use Authorization programme. The portable test will run on Abbott's ID NOW, a molecular point-of-care testing platform.
"With rapid testing on ID NOW, healthcare providers can perform molecular point-of-care testing outside the traditional four walls of a hospital in outbreak hotspots," Mr Ford said.
Abbott will supply the tests next week to healthcare providers in urgent care settings in the US. The company said it is working with the FDA to deploy tests to areas where they can have the greatest impact.
Last week Abbott received FDA approval for its m2000 RealTime SARS-CoV-2 EUA test, for use in hospital and reference labs around the world. Between the two platforms, Abbott expects to produce about 5 million tests per month.
Abbott's Covid-19 test works by taking an upper respiratory tract swab to collect mucus sample for testing, then the sample is mixed with reagents that break open the virus and release its genetic material, the viral RNA. The mixture is then put into the ID NOW system, which recognizes and replicates select sequences of the coronavirus genome, according to the company's Facebook page. The ID NOW system is small and lightweight at 3.17 kilograms, a portable box the size of a small toaster.
"We are ramping up our manufacturing capacity to the highest levels so we can supply as many tests as possible to customers in other parts of the world," the company said in response to comments on its Facebook page to supply the test outside the US.
The Chicago-based company employs 103,000 people in more than 160 countries, according to its website.